
Chirp, chirp. healing crickets.
[Editor’s note: Scott is having a very busy week, and stepping in to help out is Dr. James Coyne with another guest post.]
Bizarrely, the megajournal PLOS One has published a paper that is suspiciously similar to an advertisement. The paper LOOKS like it’s reporting on a test of a product that supposedly treats fibromyalgia, but also pain, dementia, Parkinson’s disease and more with just SOUND: basically just listening to a 40hz tone (listen to the sound here). But it’s actually junk science that was probably created just to advertise a treatment gadget. Somehow this extremely low quality paper got through PLOS One peer review, even though it prominently links to an advertisement for the gadget. That advertisement is much more blatantly pseudoscientific, as it leans heavily on a flashy, but misleading promotional TEDx video: “Music Medicine: Sound at a cellular level.”
Join me in a call for a speedy retraction! In this article, I critique the paper and show exactly why it’s obviously junky science, and share my letter sent to the senior editor of PLOS One.
The article presents results of a pre-registered randomized controlled trial (RCT) comparing Sound Oasis Vibroacoustic Therapy System VTS-1000 to a sham treatment.
As reported in PLOS One, the RCT was designed as a crucial test of the effectiveness of the device in treating fibromyalgia. Yet, standard analyses did not find a statistically significance between the active treatment that is being promoted in the video and a sham treatment constructed as a placebo. Ouch!
Technically speaking, the study found no time-by-treatment interaction. What does that mean? Patients were assessed with a battery of at least six self-report questionnaires at baseline – before any exposure to the treatment or the sham – and afterwards, post-treatment. In this case, the time-by-treatment analysis showed that the before/after differences for patients receiving the active treatment were no different than the results for patients assigned to the sham treatment.
However, reporting of the trial emphasized only differences that occurred within the active treatment group. The paper went on to declare these results:
provide preliminary evidence that gamma-frequency rhythmic vibroacoustic stimulation may decrease fibromyalgia symptoms and ease associated comorbidities, opening new avenues for further investigation of the effects of rhythmic sensory stimulation on chronic pain conditions.
What was not emphasized is that similar analyses would have shown the same effects for patients who received the sham/placebo treatment. That is a big problem for any claims for the marketing of the device.
Presenting the results in this way disregards basic rules for interpreting a clinical trial. Peer reviewers should have caught this immediately. Actually, the PLOS One Editor should have noticed this error in interpreting the trial and not even have sent the paper out for review.
A linked website calls attention to the Clinicaltrials.gov registration of the trial:
Sound Oasis VTS-1000 – Current Medical Trials The medical potential of the VTS-1000 goes beyond sleep and stress and this is seen in the use of this potent device in three clinical trials registered with the U.S. National Institutes of Health – (1) Depression: https://clinicaltrials.gov/ct2/show/NCT02685982?term=VTS1000&rank=2 (2) Fibromyalgia: https://clinicaltrials.gov/ct2/show/NCT02493348 (3) Temporomandibular Disorder: https://clinicaltrials.gov/ct2/show/NCT02427113?term=TMD+music&rank=1
This use of trial registration as suggesting some kind of endorsement by NIH is misleading. The actual trial registration page for the trial contains the standard warning:

The introduction of the article justifies the study with reference to preliminary data concerning ‘a well-established technique’ whereby sensory stimulation delivered to the skin can reduce pain.
Mechano-acoustic vibrations have been extensively used to address acute pain during orthodontic [26–28] and cosmetic procedures [29], and is a well-established technique in orthopedic practice [30] and physiotherapy to reduce muscle soreness [31–33], low back pain [34], and orofacial pain [35]. However, little is known regarding the application of vibroacoustic stimulation in the treatment of chronic pain conditions. Previous clinical studies have explored the use of rhythmic sensory stimulation to treat fibromyalgia [36–38]. Rhythmic sensory stimulation (RSS) broadly refers to the use of sensory events – including vibrotactile, auditory, and visual flickering stimuli – applied in pulsed forms with repeated short, transient stimulus events at regular intervals, or in continuous forms which generate oscillating (e.g., sinusoidal) stimulus patterns [39]
Actual studies of treatment of fibromyalgia are cited in the introduction, but they do not clearly support the efficacy of rhythmic sensory stimulation to treat fibromyalgia.
The Discussion section concedes a lack of established plausible mechanism by which the treatment should work:
There is no consensus regarding the mechanisms underlying the effects of sensory stimulation on pain.
The Discussion section attempts to neutralize the lack of differences between active and sham treatment by raising the possibility that maybe the control/comparison group is not really a sham, but another poorly understood active, effective treatment.
(Deja vu: We often see this argument when acupuncturists cannot find differences between active and sham acupuncture.)
However, the linked TEDx talk claims strong scientific support for cartoonish, simplistic explanations of miraculous effects in some dramatic case examples.
The PLOS One article is an infomercial, a key element of a marketing strategy selling unproven medical devices to persons who believe they are suffering from health conditions that are poorly understood and that have poor outcomes within conventional evidence- and science-based medicine.
The marketing campaign leaves patients and their families with the impression that a medical device that is for sale has been proven powerful and that it is similar to what is being used in prestigious hospitals.
The marketing campaign gives no indication that treatment of debilitating, complex, and poorly understood medical conditions should be conducted under medical supervision.
The American Medical Association advises against medical journals accepting direct-to-consumer advertisements for medical devices, even when the devices are approved by the US Food and Drug Administration.
The AMA does not even consider what we find in this PLOS One article: A spun clinical trial directly promoting an unproved medical device with a link to a shopping cart allowing payment and home delivery to be arranged. No cost to the company for the advertisement, only payment of the Article Processing Costs (APCs).
We must ask how senior PLOS management and lax editorial and review processes allow readers to be misled and exploited by a journal publishing such infomercials as peer-reviewed scientific articles, not paid advertisements.
But let’s immediately get this article retracted. See my letter to the Senior PLOS One Editor at the end of this article.
The article
Janzen TB, Paneduro D, Picard L, Gordon A, Bartel LR. “A parallel randomized controlled trial examining the effects of rhythmic sensory stimulation on fibromyalgia symptoms.” PLOS One. 2019 Mar 1;14(3):e0212021.
The abstract gives the basic design for the study:
A double-blind, two-arm parallel randomized controlled trial investigated the effects of gamma-frequency rhythmic sensory stimulation on fibromyalgia. We were interested in whether rhythmic sensory stimulation would promote significant changes in fibromyalgia and associated symptoms, and whether treatment effects would differ between two distinct treatment parameters.
A total of 50 patients who had been diagnosed with fibromyalgia were randomly assigned to two test groups.
One group received vibrotactile stimulation from a continuous sine wave single-frequency stimulation (40 Hz) for 30 minutes, five days per week, over five weeks, concomitant with usual care. The second group completed the same treatment protocol but received a different stimulation, consisting of random and intermittent complex wave gamma-range vibrotactile stimulation.
The two groups were evaluated before and after for self-reported fibromyalgia symptoms, pain severity and interference, depression symptoms, quality of life and sleep quality.
What could be seen as a clever but uncool spinning of the results:
Results indicated that there were statistically significant changes from baseline to post-treatment in measures of fibromyalgia symptom severity, pain interference, depression, and sleep quality. However, treatment outcomes did not differ significantly between groups.
The spin becomes explicit in the conclusion. The seemingly good news is great for advertising the device, but should have caused the reviewers and editor to cry foul.
These findings provide preliminary evidence that gamma-frequency rhythmic vibroacoustic stimulation may decrease fibromyalgia symptoms and ease associated comorbidities, opening new avenues for further investigation of the effects of rhythmic sensory stimulation on chronic pain conditions.
Basic study design
The presentation of the technical details of the study is linked to a picture of the device being marketed and even links to where it can be purchased.
Participants enrolled in the study were randomly assigned to one of two study arms (Fig 1). Both groups received 25 sessions of 30-minute rhythmic sensory stimulation over five weeks concomitant with usual care. Group one received vibrotactile stimulation from a continuous single-frequency sine wave at 40 Hz that was amplitude modulated on an 11-second cycle plus isochronous auditory stimulation with a 160 Hz tone amplitude modulated at 40Hz. Group two received vibrotactile stimulation from randomly intermittent sounds consisting of complex wave gamma-range noise with pitch peaks at 33 Hz and 45 Hz. In the initial trial study protocol, the stimulation delivered to group two was intended as a sham stimulation, however, careful spectral analysis of the stimulus conducted after study completion revealed that this was not the case. The stimulation was delivered via a portable consumer device (Sound Oasis Vibroacoustic Therapy System VTS-1000) with a built-in low-frequency transducer located at the middle back region and stereo speakers located at ear level (S1 Fig).

Clicking in the (S1 Fig) took the reader to a photo of the device prominently displayed at the end of the article and links to the sales page.
Portable consumer device (Sound Oasis Vibroacoustic Therapy System VTS-1000) equipped with a built-in low-frequency transducer located at the middle back region and two stereo speakers located at ear level. Source: https://www.soundoasis.com/products/sleep-sound-therapy-systems/vibroacoustic-therapy-system/
The intervention
The sound stimulus delivered to group one consisted of a continuous single-frequency sine wave at 40 Hz that was amplitude modulated on an 11-second cycle, plus isochronous auditory stimulation with a 160 Hz tone amplitude modulated at 40 Hz with secondary pitch peaks at 120 Hz and 200 Hz (Fig 2). The track has a 1-second fade-in and a 13-second fade-out, with an output level of -12.7db.
The comparison control condition
The second track, administered as stimulation to group two, comprised of randomly intermittent sounds consisting of complex wave gamma-range noise with pitch peaks at approximately 33 Hz and 45 Hz, and a secondary pitch peak at approximately 95 Hz (Fig 3). The sound was generated from a single hit on a percussion instrument (gran cassa), which generated a sound burst with a duration of 500 milliseconds. Each sound burst was separated by intervals generated by a random number generator producing numbers between 1-10. Each digit had an equivalent of 250 milliseconds; so that number 1 represented 250 milliseconds, number 2 represented 500 milliseconds, number 3 represented 750 milliseconds, and so on. These numbers were used to determine the intervals between each sound onset throughout the trial. The track was produced by arranging sound bursts separated by the random intervals until one minute of material was generated. This first minute of material was repeated four more times, but each of these five identical segments was set to a different tempo; the first minute played at 120 bpm, the second at 110, the third at 115, the fourth at 125 and the fifth 130.
Competing Interests
A competing interest statement discloses a conflict of interest, but an effort to manage it by keeping the conflicted author out of key decisions.
Authors T.B.J., D.P., L.P., and A.G. declare that no competing interests exist. L.B. declares a potential conflict of interest as he has served as a paid scientific consultant to Somerset Group and Sound Oasis, and receives limited royalties from the Somerset Group for the Sonic Aid series and from Sound Oasis on the sales of the Vibroacoustic Therapy System VTS-1000 device. To manage this potential conflict of interest, L.B. was not involved in patient recruitment, consenting, or data collection process, and had no direct role in the data analysis process. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Maybe LB was not involved in these activities, as stated. But how do we explain the alignment of the spinning of the null results in the PLOS One article with the needs of an advertising campaign for a ‘powerfully’ effective device? I do not think this declaration lets the authors off the hook for a nonstandard interpretation of the results.
The website
A click on one of the links in the PLOS One article takes one to the product page for the VTS-1000 Vibroacoustic Therapy System, just as pictured in the article, but with pricing and a full range of accessories.

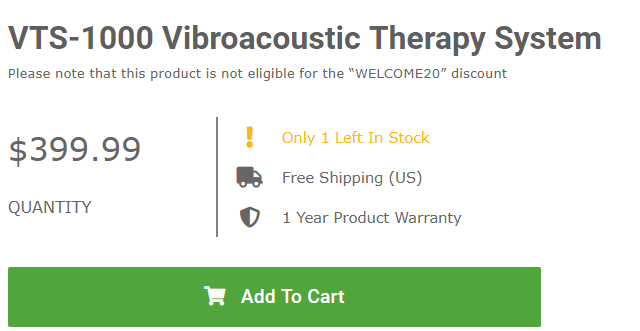
I have been visiting this site over a few weeks and the “! Only one left in stock” has been there every time.
The device is described as:
Clinically Proven, Doctor Developed Sounds…The revolutionary Vibroacoustic Therapy System uses clinically proven, doctor developed sounds and vibration to help you sleep, relax and renew your body – naturally! Vibroacoustic therapy systems, costing several thousands of dollars, have been successfully used in hospitals and clinics around the world. Now you can enjoy this same therapeutic technology at a fraction of the price. Sound therapy provides soothing music with clinically proven brainwave entrainment for effective stress reduction, relaxation and healing.
Vibroacoustic research and Dr. Bartel’s TEDx talk
The webpage allows a visitor to click directly to a TEDx talk pitch for these products:
Dr. Lee Bartel’s TEDx talk at Collingwood, Ontario gives an excellent overview of the current state of vibroacoustic medical research. Dr. Bartel explains how scientifically developed sound played through Vibroacoustic devices can help reduces the symptoms of Fibromyalgia pain, Alzheimer’s Disease, Parkinson’s Disease, Depression, and increase blood flow.
The video is a classic example of a well-polished TEDx talk pitch. Lee Bartel’s requisite moment of epiphany was extracting honey on a farm in western Canada. Honey sticking to belts made them:
Go squeak squeak squeak to drive me nuts and just as inevitably a cricket would walk into the honey house and would go chirp chirp chirp… it didn’t take very long before what started as a sort of a random chirp would synchronize with the squeak on the belt. Why this was happening was a question that lingered in my head for very many years until I discovered the principle of entrainment in physics, how one rhythmic vibrating object will synchronize with another and so I used that idea and started creating music to affect your brainwaves music…

A dreamy, satisfied woman who has just had her pain and fatigue relieved.
Years later, Bartel would discover a single pitch of 40 HZ would reduce pain in fibromyalgia and Alzheimer’s symptoms.
The video gives detailed clinical examples of seeming miraculous, enduring improvements in an Alzheimer’s and a fibromyalgia patient using this Vibroacoustic Therapy System. In the example of the fibromyalgia patient:
We gave her a prescription of 23 minutes of 40 Hertz sound stimulation two times a week for five weeks on this device…and what we found after five weeks was that she had stopped using all her medication. She’s reported that she could sleep much better and she was less depressed. The chiropractor checked her neck and shoulders and she had more mobility.
The effects of the sound stimulation on a patient with are given a simple explanation:
Research shows that fibromyalgia has connectivity issues between parts of the brain and then the pain circuits so the theory would be in our assumption was that 40 Hertz sound stimulation would restore this connectivity and she demonstrated that there were positive results.
The TEDx talk ends with a rousing promise of this powerful music medicine at the cellular level being integrated into routine, conventional medicine.
I could not have foreseen that the idea that came from this cricket could start to create sound that might impact cells in your body. That stimulating cells with sound can reduce the risk and impact of health problems like Alzheimer’s and Parkinson’s and fibromyalgia and depression. But what I do foresee now in the not-too-distant future is that when a doctor encounters something like Alzheimer’s or Parkinson’s or depression they might take out their prescription pads and write a prescription for sound stimulation and that’s music medicine at the cellular level [Applause] [Music] [Applause] [Music]
The letter
I have written to Dr. Joerg Heber ([email protected]) Editor-in-Chief of PLOS One. If readers share my concerns, they might want to tell him directly.
Dear Dr. Heber:
I am writing to request a formal investigation of an article that appeared in PLOS One. I believe that a prompt retraction would be in the interest of the reputation of the journal and would protect consumers from the marketing of a dubious unproven medical device claiming dramatic effects of a 40 Hz sound on Fibromyalgia pain, Alzheimer’s Disease, and Parkinson’s Disease.
The article is
Janzen TB, Paneduro D, Picard L, Gordon A, Bartel LR. A parallel randomized controlled trial examining the effects of rhythmic sensory stimulation on fibromyalgia symptoms. PLOS One. 2019 Mar 1;14(3):e0212021.
The PLOS One article reports a RCT in which no differences were found between the active treatment of rhythmic sensory stimulation of 40 Hz Vibroacoustic Therapy System and a cleverly constructed sham treatment. Despite this being a null trial, the article focuses on the within-differences for the active treatment (no different than in the sham group) and declares the results
provide preliminary evidence that gamma-frequency rhythmic vibroacoustic stimulation may decrease fibromyalgia symptoms and ease associated comorbidities, opening new avenues for further investigation of the effects of rhythmic sensory stimulation on chronic pain conditions.
The PLOS One article contains a picture of the device that is being marketed direct to consumers and the caption to the picture is redundant with another link going directly to a product page where the VTS-1000 Vibroacoustic Therapy System is offered for $399.99, but with the admonition that there is only one device left in stock.
The website also prominently displays a TEDx talk with outlandish claims that I recommend you view.
The PLOS One article is an infomercial, part of a crass marketing of an unproven medical device to vulnerable patients and their families struggling with managing complex and poorly understood medical conditions for which conventional evidence- and science-based medicine do not offer much hope of relief.
AMA advises against medical and scientific journals accepting advertisements for medical devices, even those with FDA approval. PLOS One is now in the position of marketing an unproven device for the relatively low cost of APCs.
I hope the journal will respond promptly to this alert. I recognize that policing the quality of articles in a megajournal with ~20,000 articles published per year is a formidable task. But only a few, or even one articles displaying a few breakdowns in editorial and peer review can have substantial impact on authors and lay and professional perceptions of the trustworthiness of the journal. A prompt investigation to determine whether this article should be retracted or merely have a correction could be an important step in limiting any damage. I obvious favor a retraction.
Please let me know if I can provide further information.
Sincerely,
James C. Coyne, PhD
Note
Thanks to Paul Ingraham of PainScience.com for editorial assistance. I highly recommend his science-based fibromyalgia tutorial.

